
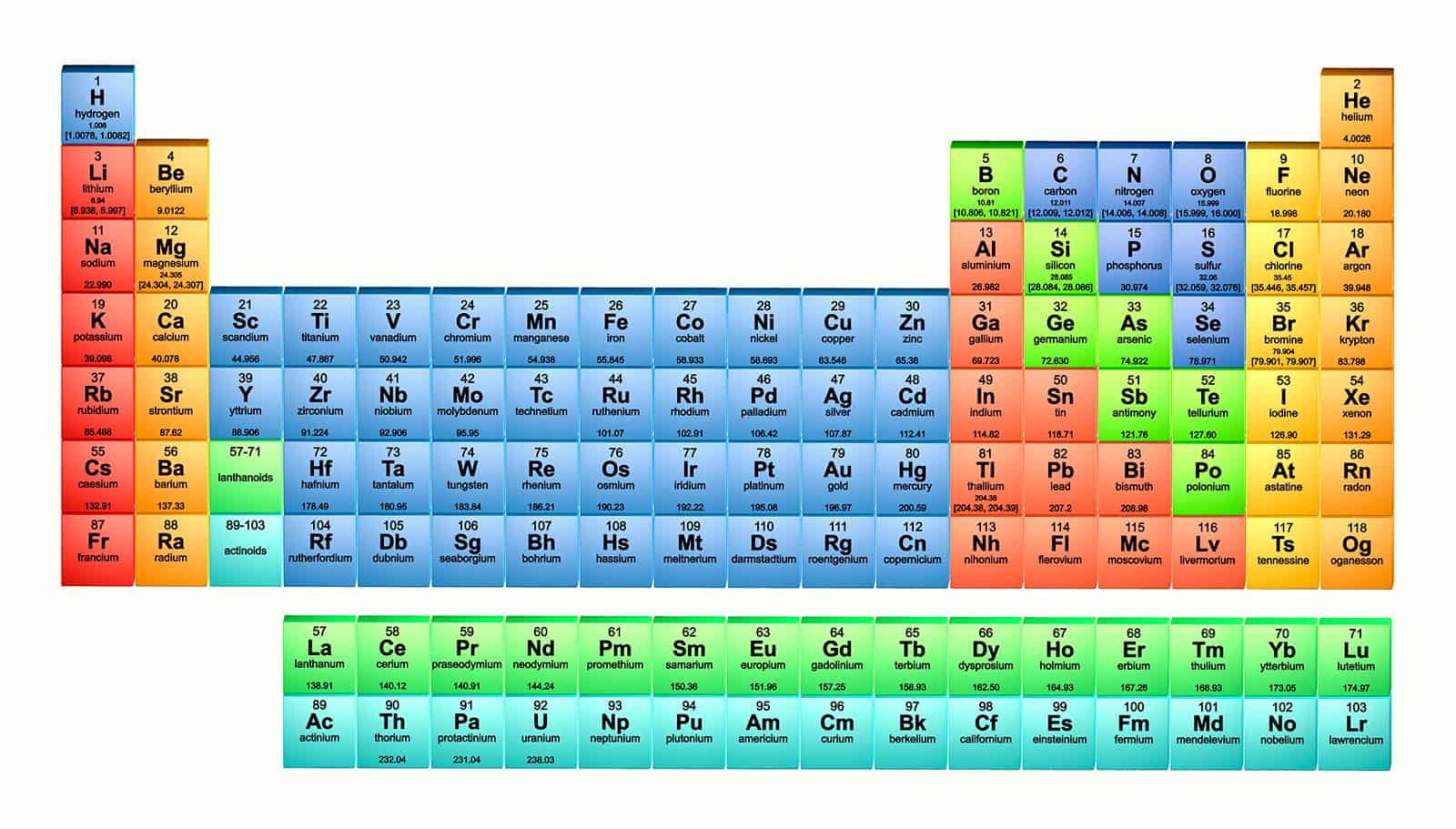
However, only 114 of these elements are officially recognized by the scientific community. These two new elements now make the number of elements displayed on the periodic table 117. These aren't elements you'll likely find out in nature, but they are elements nonetheless and now have proud places on the periodic table. They are lab-created elements that scientists made as side effects of smashing the atoms of other elements together to see what they would do. Mendeleev's periodic table was the first in rows and columns.Image Credits: Atomic Structureįlerovium and livermorium are short-lived elements, each lasting only seconds or less. Polyamide 6 - Nylon 6 - PA 6 20% PTFE Lubricated.CRAIC Introduce Automated Version of 20-20 Perfect Vision UV-Visible-NIR Microspectrophotometer.Zero 20/20 Project – Ground-Breaking Energy Retrofit.These elements are flerovium (element 114) and livermorium (element 116). For example, two brand new elements were discovered in 20 respectively, and added to the periodic table in 2012. However, some interesting and significant changes have been made as recently as the past 20 years. The periodic table is continually being changed as new discoveries are made and new theories are developed to explain the behavior of chemicals.Ī huge number of changes were made to the periodic table in the early parts of the 20th century. It has even changed the weights of other elements. The periodic table has long-since filled in Mendeleev's gaps and has added new elements. Mendeleev predicted that the discovery of some as-yet unknown elements would fill in some of the gaps on his table at some point in the future, and he was correct. It included the properties of all of the known elements of that time. The first periodic table in the "rows and columns" form we see today was invented by Dmitri Mendeleev in 1869.

The periodic table is much more fluid than the majority of people realize. Most people have been seeing the periodic table on classroom walls since grade school, and most people probably think it never changes. The elements are shown according to their increasing atomic weights, starting with the smallest and gradually moving up to the highest weights. The organization on the table is based on the atomic weight, electron configurations, and chemical properties of the elements. The periodic table is a chart that organizes the arrangement of the known and recognized chemical elements.


 0 kommentar(er)
0 kommentar(er)
